
EMA Marketing Authorization of New Drugs in March 2025
Shots:
-
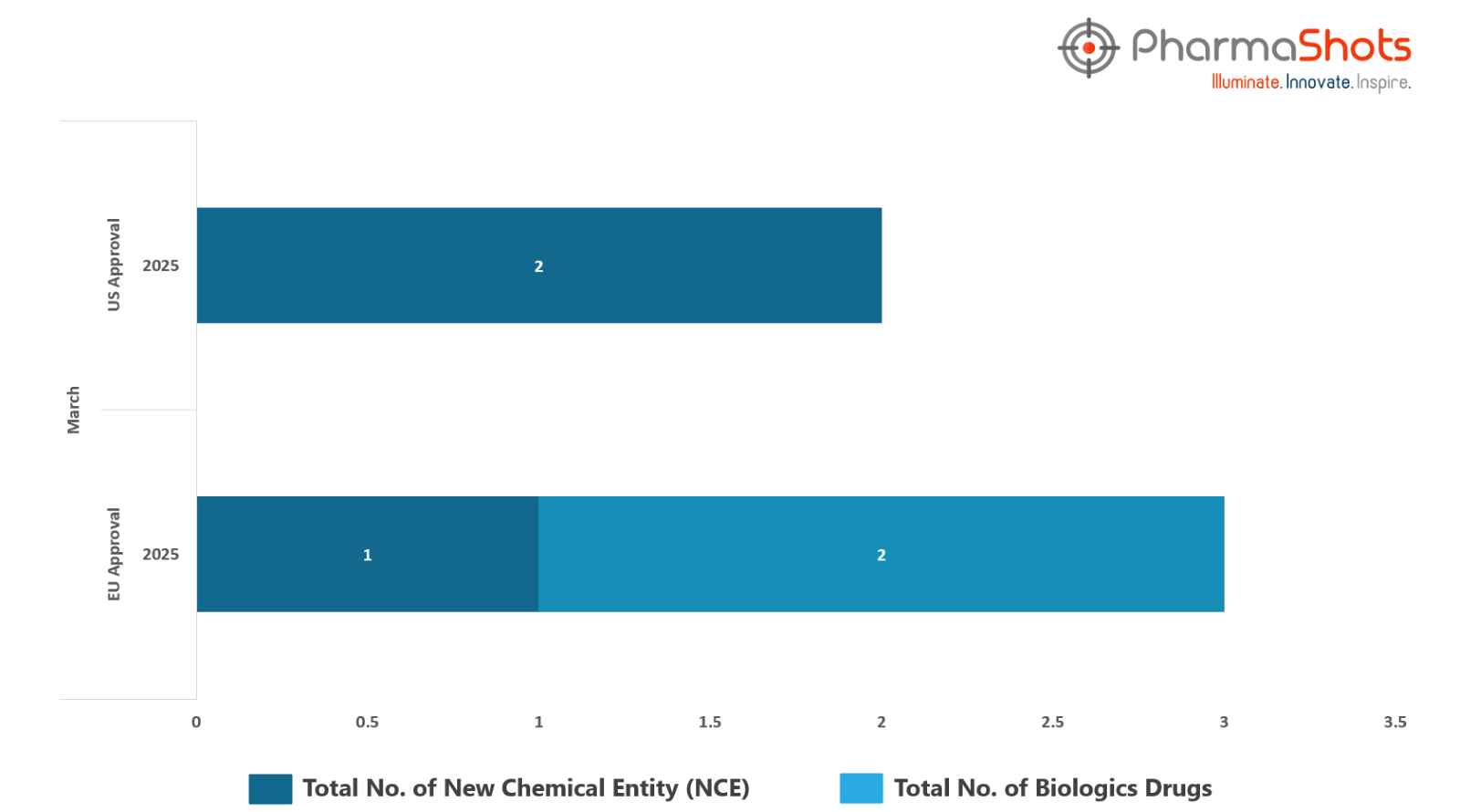
The EMA’s CHMP has granted positive opinions and approvals to 2 Biologics and 1 New Chemical Entity in March 2025, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were Merck’s Capvaxive to treat invasive pneumococcal disease
-
PharmaShots has compiled a list of 3 drugs that have been granted positive opinions and approvals by the EC, respectively
Company: Merck
Product: Capvaxive
Active Ingredient: Pneumococcal 21-valent Conjugate Vaccine
Disease: Pneumococcal Disease
Date: Mar 26, 2025
Shots:
-
The EC has approved Capvaxive for active immunization against invasive disease & pneumonia caused by S. pneumoniae in adults (≥18yrs.) in 30 EEA states
-
Approval was backed by P-III (STRIDE-3) data, assessing Capvaxive vs PCV20 in pneumococcal vaccine-naïve adults (≥18yrs.), plus data from various P-III trials (STRIDE-4, STRIDE-5, STRIDE-6, STRIDE-7, & STRIDE-10) assessing it in both vaccine-naïve & vaccinated adults
-
STRIDE trials showed Capvaxive was non-inferior/superior to PCV20, PPSV23, & PCV15 by OPA GMTs, with strong immune responses for all serotypes. It also maintained efficacy in HIV pts (STRIDE-7), with QIV (STRIDE-5), & in previously vaccinated subjects (STRIDE-6 & 7)
2. Averoa Receives the CHMP’s Positive Opinion for Xoanacyl to Treat Chronic Kidney Disease
Company: Averoa
Product: Xoanacyl
Active Ingredient: Ferric Citrate Complex
Disease: Chronic Kidney Disease
Date: Mar 27, 2025
Shots:
-
The CHMP has recommended Xoanacyl (ferric citrate complex) for chronic kidney disease, with an EU decision expected by Jun 2025, while the UK's MAA will be filed via MHRA's IRP, with decision anticipated in the coming mos.
-
MAA was filed via centralized European procedure in Mar 2024 & was supported by 3 pivotal trials conducted by Akebia Therapeutics
-
Averoa licensed Xoanacyl from Akebia in Dec 2022, enhancing its dossier with a re-engineered clinical package to support dual indications for EU pts. Averoa is currently seeking commercial partners for EU launch & distribution
Company: Genmab and Pfizer
Product: Tivdak
Active Ingredient: Tisotumab Vedotin
Disease: Recurrent or Metastatic Cervical Cancer
Date: Mar 31, 2025
Shots:
-
The EC has approved Tivdak an ADC, as a monotx. for adults (n=502) with recurrent or metastatic cervical cancer with disease progression on or after systemic therapy
-
Approval was backed by P-III (innovaTV 301) global trial evaluating Tivdak vs CT alone (topotecan, vinorelbine, gemcitabine, irinotecan, or pemetrexed)
-
The trial met its 1EP of OS, showing a 30% reduction in death risk with vs CT. Median OS was 11.5mos. v. 9.5mos. CT. 2EPs of PFS & ORR were also met, with a 33% reduction in the risk of disease progression vs CT. Data from the innovaTV 204 P-II trial evaluating Tivdak as monotx. in previously treated recurrent or metastatic cervical cancer was included in the MAA
Related Post: Insights+: EMA Marketing Authorization of New Drugs in February 2025
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














